Last updated on 2025-11-07 at 11:23 EST (UTC-05:00)
Today was the second class in the RAC Amateur Radio License course. We were studying Chapter Two of the Study Guide, Introduction to Electronics.
This chapter lays the foundation for understanding basic electricity, beginning with the fundamental components of matter. Before delving into the technical theory, Al discussed the Amateur Radio Operator Code of Conduct. I decided that the two codes Al mentioned were important enough to warrant their own post, so you can find them here.
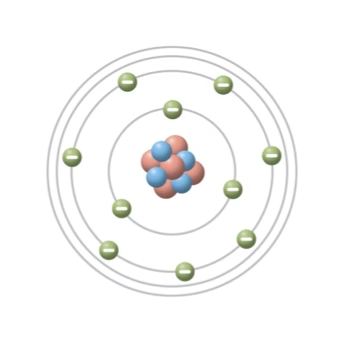
We looked at atoms, their structure, and how the behaviour of electrons gives rise to electrical phenomena. From there, the discussion moved into conductors and insulators—why materials like copper and gold conduct electricity so well, while glass, rubber, and plastics resist it.
Permittivity is a key idea in physics, especially in electromagnetism. It describes how a material reacts when an electric field is around. Understanding permittivity helps explain how electric fields work, as well as the functioning of capacitors, dielectrics, and electromagnetic waves.
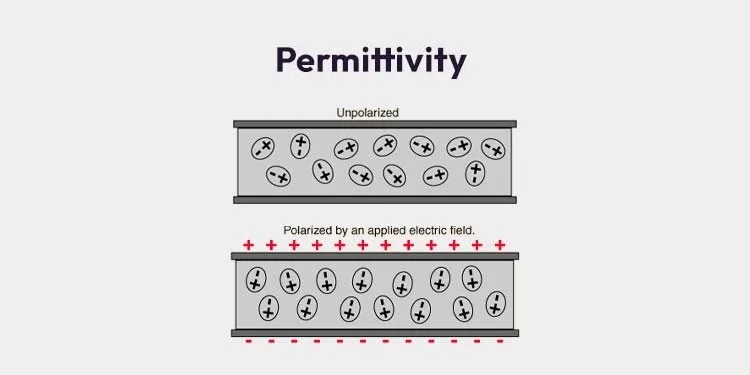
Permittivity, denoted by the symbol ε (epsilon), is essentially a measure of how well a material can allow electric fields to pass through it. It tells us how much the electric field inside the material is weakened compared to what it would be in a vacuum or open space. This property depends on the material’s makeup, structure, and physical state.
Key ideas:
- Electric Permittivity (ε0): This is the permittivity of free space, often called epsilon naught. It’s a fundamental constant that describes how electric fields behave in a perfect vacuum. Its value is about 8.854 × 10-12 farads per meter (F/m). In a vacuum, the electric field moves without distortion or loss.
- Relative Permittivity (εr): Also known as the dielectric constant, this is simply the ratio of a material’s permittivity to that of free space. It’s dimensionless and tells us how well a material can store electrical energy in an electric field compared to a vacuum. Materials like glass, ceramics, and many plastics have high relative permittivity, making them very effective at energy storage.
Insulators or insulating materials are those substances which will not allow the flow of electrons through them due to very low free electrons in them, and they have a low dielectric constant (Relative permittivity = εr).
Examples: Porcelain insulators used in power transmission on distribution poles and towers, rubber, glass, plastic, wood, etc.
Dielectrics or dielectric materials are substances similar to insulators but allow the flow of electrons through them when subjected to an external electric field, as they can be polarized. They can also be defined as having the ability to store charge (energy) through polarization, as in a capacitor. Additionally, they have a high dielectric constant. (Relative permittivity = εr).
Examples: A common example of a dielectric is the electrically insulating material between the metallic plates of a capacitor, (such as mica, laminated paper). Other examples include air, ceramic, etc.
- All dielectrics are insulators, but not all insulators are dielectrics.
- Everything becomes a conductor at certain temperatures or electric fields due to breakdown, as every insulator has its limits to withstand a potential difference across the material.
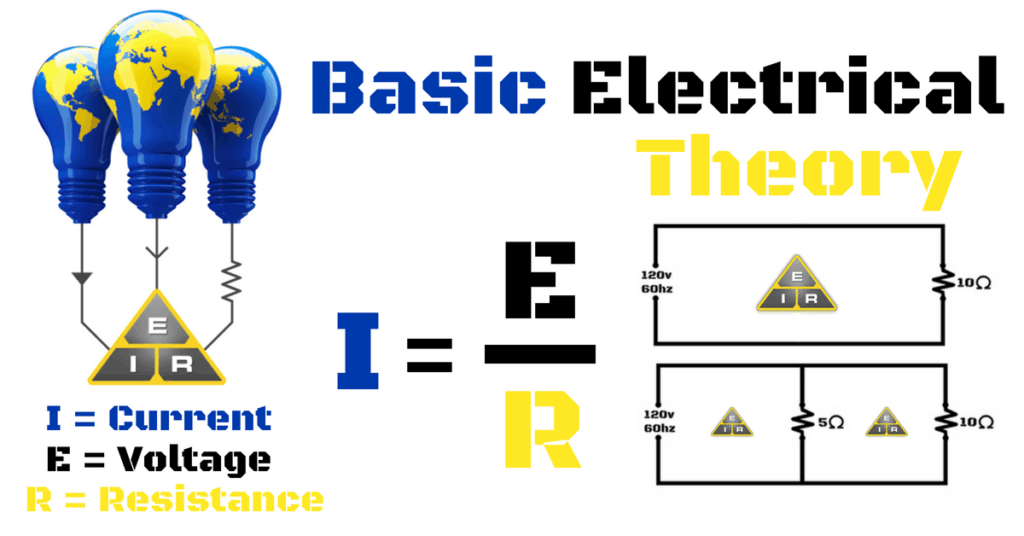
Key electrical concepts were introduced, including charge, current, voltage, and resistance. Al Penny VO1NO, our instructor, explained the coulomb as the standard unit of charge, the ampere as the rate of electron flow, and voltage as the “pressure” that pushes electrons through a conductor. Resistance and the factors that affect it—material type, length, diameter, and temperature—are also covered, along with the role of resistors and potentiometers in circuits.
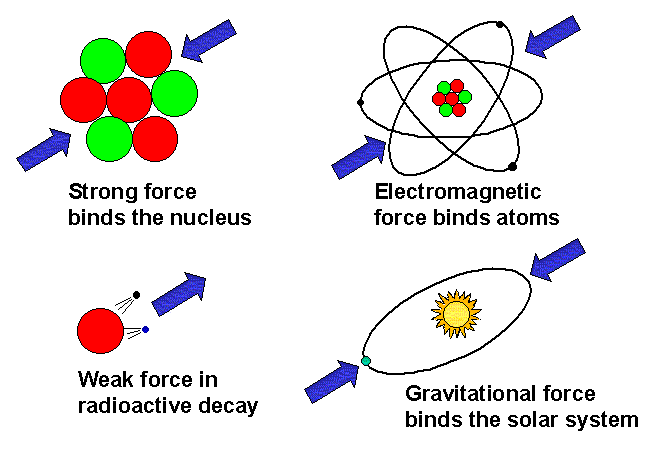
The class then explored magnetism as one of the four fundamental forces of nature, showing how magnetic fields, poles, and materials influence electrical behaviour.
This naturally led to a discussion of direct current (DC), its sources, and the role of cells and batteries. Al explained the difference between primary (non-rechargeable) and secondary (rechargeable) cells, the chemistry behind common examples like zinc–carbon and lead–acid batteries, and how cells can be connected in series or parallel to change voltage or current capacity.

By the end, the chapter tied together the essential elements of electricity—atomic theory, conductors and insulators, current, voltage, resistance, magnetism, and electrochemical cells—providing a solid grounding for anyone beginning their journey into radio and electronics.
This was a review for me, as I have worked with electronics and electricity throughout my career. However, for anyone who doesn’t have a grounding in these subjects or feels like they need a refresher, this was a great place to start.

If you are thinking about studying for an Amateur Radio Certificate, there are some excellent flashcard decks available for free on Ankiweb. One that I am using is the ISED basic amateur questions (2025) deck.


Leave a Reply